
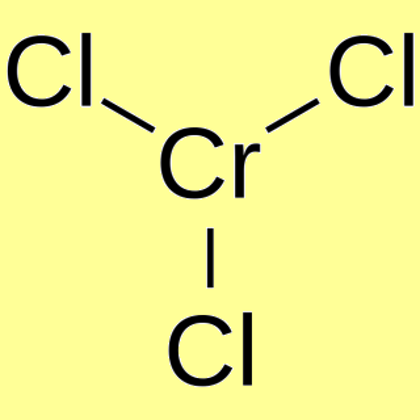
That brings us to to this very important question, what is the significance of the water in the ionic sphere? Now most books say that it's the water of crystallization of that compound. So that would mean that this exists and since it is theoretically an isomer, it should also be an isomer practically. Now, it's also believed that the H2O is actually held to the central metal atom (Cr) with the help of dipole forces and that the voids are not needed. Therefore this isomer doesn't exist and hence cannot be the 4th isomer. But here there isn't any anion in the ionic sphere, in fact it is believed that there isn't any ionic sphere. Now if the isomer 3H2O should exist then it must have these voids. The water of crystallization is believed to be present within the voids created by an anion and a cation. The H2O that exists outside the coordination sphere is the water of crystallization. The reason given by the authors to support there claim of only 3 isomers is not very concrete, but it still majority of the authors believe that it is true. Some say there are 3 or rather most of them say 3, only one author, JD Lee says that there are 4. Your question on CrCl3.6H2O is a very debatable topic and there hasnt been a unanimous decision that has been put forth by all the authors. Disposal considerations Waste Disposal Methods Chemical waste generators must determine whether a discarded chemical is classified as a hazardous waste.

But in this case, where the two spheres carry no charge, there isn't a force, namely the electrostatic force, which binds the two spheres. Chromium(III) chloride, anhydrous Revision Date 2 Component log Pow Chromic chloride -3 13. The two coordination spheres should act as individual ions and hence participate in the ionic sphere. Chemical Class: The hexahydrate of chromium(3+) trichloride.Regarding the coordination isomerism question, the reason why it's not considered as an isomer in the case of two uncharged complexes, is that there is no ionic attraction.OU Chemical Safety Data (No longer updated) More details Safety glasses, gloves, good ventilation.Treat as a possible carcinogen. The recovered chromium chloride weighed about 119 grams which was about 72.3 of the theoretical yield. Whats the theoretical percentage water in Chromium (III) chloride hexahydrate ISBN: 9781305079250 Author Mark S. Computed by PubChem 2.1 (PubChem release 2021.05. The slurry of chromic chloride was filtered through a fritted glass filter. NOTE: The chromium(III) chloride hexahydrate. The concentration of all solutions should be such that the low energy absorbance maxima is between 0.2 and 1.5 absorbance units. Toxicity: ORL-RAT LD50 1790 mg kg-1, IPR-MUS LD50 520 mg kg-1 OU Chemical Safety Data (No longer updated) More details Chromium(III) chloride hydrate, 99.995 Chromium chloride (CrCl3), hydrate (1:6) PubChem. As the analysis of o only requires that max be identified, you can use approximately 10 mg of each complex in about 5 ml of solvent.It is suitable as a raw material for various chromium-containing catalysts. During my early chemical investigations, I acquired some chromium(III) chloride hexahydrate, a green salt that gave an equally green solution when dissolved in water. This medicine may become less effective after 1 or 2 weeks of use. OU Chemical Safety Data (No longer updated) More details Chromium(III) Chloride Hexahydrate - Introduction. Chloral hydrate should be given only for a short time. Hygroscopic.Incompatible with lithium, nitrogen, strong oxidizing agents. Stability: Stable, but may be air-sensitive.Appearance: greenish-black to violet crystals OU Chemical Safety Data (No longer updated) More details.Experimental Solubility: Slightly soluble in acetone.Experimental Melting Point: 83 ☌ Alfa AesarĨ3 ☌ OU Chemical Safety Data (No longer updated) More details (The most commonly used salt is chromium(III) chloride hexahydrate, which is actually the chloride salt of the trans isomer of the CrCl 2 (H 2 O) 4 + cation.Experimental Physico-chemical Properties.


 0 kommentar(er)
0 kommentar(er)
